

This lifts the material in the top sections of the bed causing loss of solid material and very poor efficiency.įluid beds produce large levels of acoustic emission. In extreme cases when the bubble diameter approaches that of the vessel a phenomenon known as ‘slugging’ may develop. When bubbles grow too large they serve as conduits for gas by-pass thereby reducing gas–solid contact. The bubbles are responsible for much of the mass transport of particles and are an important part of the mixing process. In the wake of these pockets is a stream of high-velocity particles. These are pockets of gas moving upward through the bed.
In dense beds a ‘bubble phase’ is often seen. The particles move upwards in the center of the bed and fall downwards, under gravity, at the walls. In a fluidized bed, an upward current of gas suspends particles. These are of particular interest in the food and pharmaceutical industries. The gas is the monomer ethylene or propylene.įluid beds are frequently used to produce granules or to dry particulate materials. The particles consist of growing polyolefin granules (polyethylene or polypropylene). In the petrochemicals industry they are used for producing polyolefins on a very large scale.

Belchamber, in Encyclopedia of Analytical Science (Second Edition), 2005 Fluid Bedsįluid bed technology is important in the oil, petrochemical, pharmaceutical, and food industries.Ĭatalytic cracking is an important process in the oil industry where petroleum vapor passes through a low-density bed of catalyst, which causes the heavier fractions to ‘crack’ producing lighter more valuable products. Sometimes, hydrogen is also produced during cracking.R.M. Some of the smaller hydrocarbons formed by cracking are used as fuels (eg large chains are often cracked to form octane for petrol, which is in high demand), and the alkenes are used to make polymers in the manufacturing of plastics.

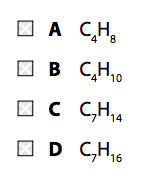
There is a greater demand for smaller hydrocarbons than larger ones. Cracking produces smaller alkanes and alkenes. These processes break covalent bonds in the molecules, causing thermal decomposition reactions. passed over a catalyst of silica or alumina.Fractions containing large hydrocarbon molecules are heated to vaporise them. This is where cracking comes in.Ĭracking allows large hydrocarbon molecules to be broken down into smaller, more useful hydrocarbon molecules. Crude oil often contains too many large hydrocarbon molecules and not enough small hydrocarbon molecules to meet demand. Fuels made from oil mixtures containing large hydrocarbon molecules are not efficient as they do not flow easily and are difficult to ignite.


 0 kommentar(er)
0 kommentar(er)
